Kazimierz Wielki University in Bydgoszcz, Poland
Introduction and purpose: In ancient times, cannabis was used for production of food, edible oil, fibers and medicines. In present, industrial cultivars are the source of raw material for clothing and animal feed. Cannabis can be also a source of psychoactive compounds such as 9-tetrahydrocannabinol (THC). Cannabinoids present in Cannabis sp. can cause the beneficial effects in human organism after consumption. This review is aimed to describe the biological properties of cannabis and consider the legalization of marijuana products.
Brief description of literature: The endocannabinoid system consists of cannabinoid receptors (CB1 and CB2) and endogenous cannabinoids. CB1 receptors are mostly localized in central nervous system. These receptors are found primarily on central and peripheral neurons in the presynapse. These locations facilitate their inhibition of neurotransmitter release, which is one of the major functions of the endocannabinoid system. CB2 receptors are mostly localized in immune system cells. The interaction of plant cannabinoids with specific receptors can cause several effects in organism. The tissue distribution of CB1 and CB2 is responsible for the well-known psychotropic and peripheral effects of THC. Cannabinoids can be also helpful in treatment in several diseases, such as multiple sclerosis, Tourette syndrome, eating disorders, chronic pain or epilepsy.
Conclusions: Despite well-documented health effect of marijuana using, cultivation of cannabis is highly restricted by the law. The main obstacles limiting the use of marijuana in medicine are the psychotropic side effects. In this case, the compromise is difficult to achieve. In future, researchers must cooperate to develop orally administered, highly bioavailable, non-psychoactive phytocannabinoid products.
Keywords: Cannabis, 9-tetrahydrocannabinol, endocannabinoids, cannabinoid receptors, medical marijuana
Introduction
The genus Cannabis includes three major species: C. sativa, C. indica and C. ruderalis. Cannabis is classified as herbs and has long thin flowers (Anwar et al. 2006, Vonapartis et al. 2015). This plant has been known for thousands of years (Callaway 2004). It was cultivated all over the world until the early twenties when cultivation of this plant was forbidden. At that time, cultivation of plants that were a source of 9-tetrahydrocannabinol (THC), a psychoactive substance, were banned (Galasso et al. 2016). The oldest reports on the use of cannabis for medicinal and nutritional purposes are found in traditional Chinese medicine (Callaway 2004). This plant is native to Central Asia. For thousands of years, it has been cultivated as a source of food, edible oil and raw material for the production of fibers and medicines. In recent times, hemp has become a valuable source of fibers used in clothing and animal feed (Liang et al. 2015). The cannabis stem allows to produce very durable fabrics and special purpose papers such as canvas, tea bags, paper money or cigarette papers (Callaway 2004).
Nowadays, cultivation of Cannabis sp. is highly restricted by the law. The European Union allows for the cultivation of only industrial varieties that contain less than 0.3% THC. THC is the most recognizable compound in cannabinoids group. Due to its relatively high content in some parts of cannabis, this plant has strong psychoactive properties. The most THC-rich part of cannabis plant are female flowers, that after drying and eventual fermentation are called marijuana. In addition to THC, cannabis contains over 80 other cannabinoids, including cannabidiol (CBD), cannabinol (CBN), and others. Industrial cannabis varieties are characterized by higher levels of CBD than THC. Differences in chemical structure of main representatives of this group are presented in Fig. 1.
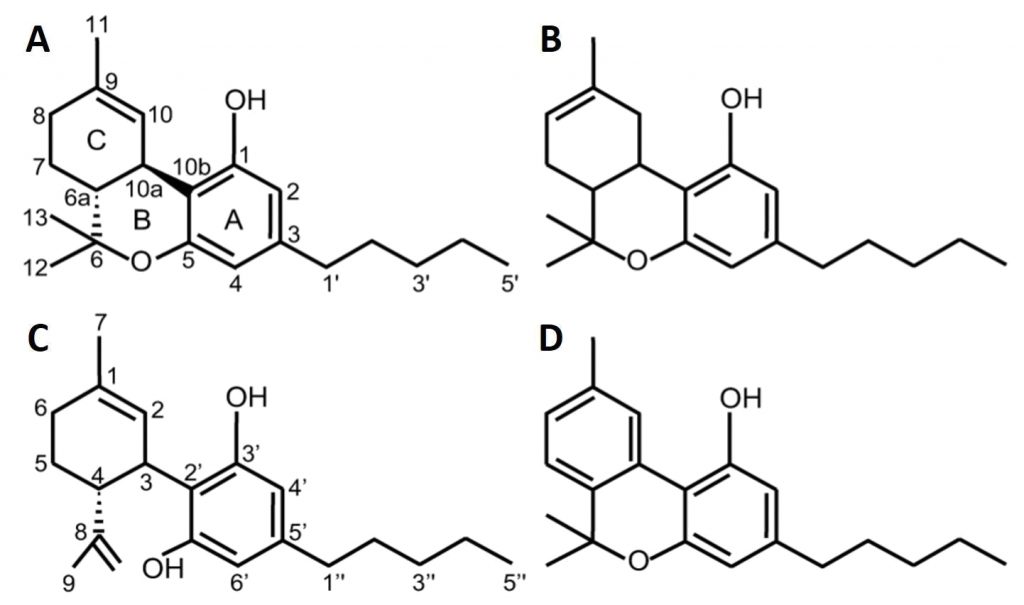
Fig. 1. The chemical structure of main cannabinoids present in Cannabis (A – 9-THC; B – 8-THC; C – cannabidiol; D – cannabinol) (Pertwee 2005).
The current debates on cannabis are aroused mainly by ignorance of the facts about the plant and its bioactive compounds. Almost one thousand research articles and a host of well-documented reviews have been written in the last three decades on this type of compounds and their biomedical relevance (Appendino et al. 2011).
There is social dispute between two groups of people: some disapprove the therapeutic applications of cannabis products, others want the legalization of marijuana for medicine. First group explain their opinion due to the potentially harmful acute and short-lasting effects of psychoactive compounds. The possibility of induction psychological side effects, such as psychosis or depression as well as dependency concerns are often repeated arguments by antagonists (Patton et al. 2002).
Every decision (ban or legalization) must be preceded by knowing the full mechanism of cannabinoids activity in human body. The aim of this study is presentation of the mechanism of biological activity of cannabinoids for easier consideration the legitimacy of legalization medical products obtained from marijuana.
The endocannabinoid system
The endocannabinoid system consists of cannabinoid receptors (CB1 and CB2) and endogenous cannabinoids (endocannabinoids) (Siudem et al. 2015).
Receptors
CB1 and CB2 belong to the superfamily of G protein–coupled receptors (GPCRs). G-protein is biological membrane protein present in eukaryotic organisms. Both receptors have 48% amino acid sequence identity. They are bound through G proteins to adenylyl cyclase and mitogen-activated protein kinase. These receptors are mostly localized in central nervous system (CNS) primarily on central and peripheral neurons in the presynapse. CB1 is one of the most common GPCR. Its density is highest in the basal ganglia, cerebellum, hippocampus and cortex. However, we can find this receptor also in the peripheral nervous system (PNS) and several peripheral organs. These locations facilitate their inhibition of neurotransmitter release, which is one of the major functions of the endocannabinoid system (Mechoulam and Parker 2013). It is possible because of the preferential distribution of CB1 receptors in CNS and PNS at presynaptic neurons, their coupling to the inhibition of voltage-activated Ca2+ channels, and the stimulation of endocannabinoid formation by increased intracellular Ca2+ and activation of other GPCRs (Di Marzo et al. 2004). When CB1 receptors are activated, the accumulation of cyclic adenosine monophosphate (cAMP) decreases and causes inhibition of cAMP-dependent protein kinase (PKA). On the other hand, activation of CB1 receptor stimulates the mitogen-activated protein (MAP) kinase activity. Increased activity of this enzyme allows cannabinoids to modulate synaptic plasticity, cell migration, and possibly neuronal growth (Howlett et al. 2002).
Occurrence of CB2 receptors was originally linked only with immune system cells. However, these receptors are present throughout the central nervous system (Ashton et al. 2006). In several pathological conditions, expression of CB2 receptor is enhanced in the central nervous system as well as in other tissues (Mechoulam and Parker 2013). This phenomenon may indicate that CB2 receptor is one of the constituents of general protective system. Immune system of mammalian is adjusted for acting against protein attacks. It is targeted for preventing, attenuating or repairing the inflicted damage. Evolution led to evolve analogous biological protective systems against nonprotein attacks. The part of such system can be endocannabinoid signaling through CB2 receptors (Pacher and Mechoulam 2011).
Cannabinoids
The presence of cannabinoid receptors implies the existence of endogenous ligands. Cannabinoids that can be specific for CB1 and CB2 receptors can be divided in 2 main groups: plant cannabinoids (phytocannabinoids) (Fig.1) and endogenous cannabinoids (endocannabinoids) (Fig. 2).
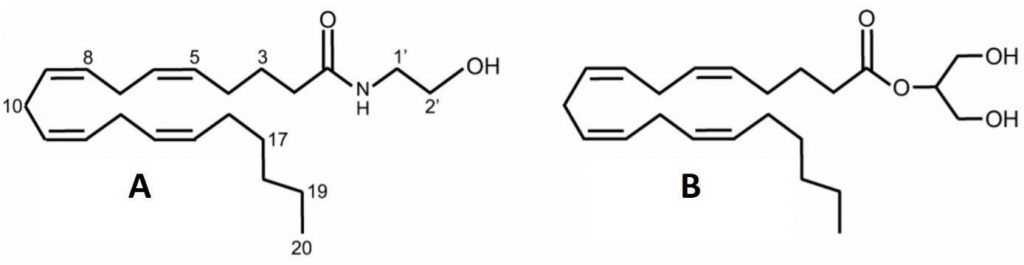
Fig. 2. The chemical structures of endocannabinoids (A – anandamide, B – 2-arachidonoyl glycerol (2-AG)) (Pertwee 2005).
Two main endocannabinoids – anandamide and 2-arachidonylglycerol (2-AG)3–5 – are lipids in nature. However, these compounds are different not only in the case of their chemical structures, but amino acid, amine and peptide transmitters, too. The transmitters are synthesized in the cytosol and stored in synaptic vesicles. Nerve excitation by action potentials cause their secretion by exocytosis. By contrast, anandamide and 2-AG can be produced upon demand by receptor-stimulated cleavage of membrane lipid precursors and released from cells immediately after their production (Piomelli et al. 2000).
The tissue distribution of CB1 and CB2 is responsible for the well-known psychotropic and peripheral effects of phytocannabinoids. The most important in this group are 9-THC and cannabidiol. These compounds are differentiated by the character of their interactions with receptors. THC acts as a cannabinoid receptor agonist, while the CBD as an antagonist. The CBD has antipsychotic properties, has a beneficial effect on anxiety and depression, which is probably related to serotonergic effects. The CBD also has sedative and anticonvulsant effects (Siudem et al. 2015). Mechanism of action of cannabidiol in organism has not yet been fully discovered. It is currently suggested that it has a weak affinity for cannabinoid CB1 receptor, and stronger for CB2 and adenosine A2A receptor (Burstein 2015). As is mentioned above, cannabinoid receptors are bound to the G-protein. The interaction of CBD with the receptor causes protein G activation and initiation of biochemical pathways (Welty et al. 2014). It has also been observed that the CB1 receptor and the serotonin 5HT1A receptor may form hetero-dimers (Pazos et al. 2013). Cannabinoids also interact with such dimers. Another molecular target for CBD is the TRPV vanilloid receptor, which regulates intracellular calcium ion concentration. It has also been observed that CBD is a GPR55 receptor antagonist (Siudem et al. 2015).
Therapeutic effects of marijuana
Consumption of cannabinoid-rich parts (especially the resin) of cannabis plant can help to reduce multiple sclerosis (MS) symptoms, such as spasticity, neuropathic pain, tremor, and disturbed bladder function. Multiple sclerosis is dysfunction that induce pathological changes in endocannabinoid system. It was shown that an antispastic effect of cannabinoids in the mouse model with multiple sclerosis is mediated primarily by CB1 receptors. This phenomenon can be explained by experimental autoimmune encephalomyelitis (EAE) model. In study of Pryce and Baker (2007) the antispastic effect was observed following administration of nonselective CB1/CB2 receptor agonists in wild-type mice with EAE, whereas the effect was no longer detected in CB1 receptor-deficient mice. It can be concluded, that the administration of THC and CBD in a 1:1 ratio has proven to be a well-tolerated antispasticity therapeutic solution for patients with MS who respond poorly to conventional antispastic drugs (Leussink et al. 2012).
There are several studies concerning cannabinoids usage in chemotherapy as the antiemetics (Tramèr et al. 2001). Randomized trials showed that patients preferred cannabinoids for future chemotherapy. This fact is important because usage of these compounds was only slightly more effective than conventional antiemetic and only for moderately emetogenic chemotherapy. In this case preferences of patients are more important than the scientifically obtained efficacy of cannabinoids use. Side effects coupled with cannabinoids occur more often, but they may be concentrated in a fairly small number of patients. This situation can be a reason why most patients find cannabinoids effective without adverse effects. Some side effects can be even beneficial. In study of Tramèr et al. (2001) among 100 cancer patients who received a cannabinoid, 30 of them were sedated, 20 felt a sensation of a “high”, and 15 felt euphoric. Some part of patients perceived a degree of sedation or somnolence as useful during chemotherapy.
In some cases, cannabinoids uptake can reduce symptoms in Tourette syndrome (TS) (Hemming and Yellowlees 1993, Müller-Vahl et al. 2002, 2003, Müller-Vahl 2013). As mentioned above, large amount of cannabinoid receptors are located in basal ganglia and hippocampus. This indicates that cannabinoids demonstrate significant role in movement and behavior. Some reports suggest beneficial effects of marijuana use in TS. It can be speculated that cannabinoids might act through specific receptors, and that the cannabinoid system might play a major role in TS pathology. Second mechanism causing amelioration in TS can be interaction between cannabinoid and dopamine receptors in basal ganglia. There are some studies that confirm the ability of cannabinoids to alter dopaminergic neurotransmission in brain and influence the motor control (MüllerVahl et al. 1998, Lazenka et al. 2015). Analyzing available data basing on several single case studies and some small controlled trials it can be concluded, that cannabinoids may play beneficial role in the treatment of tics and coprolalia in patients with TS (MüllerVahl et al. 1998).
Endocannabinoid system plays significant role in appetite regulation. It is well documented that it participates in both the homeostatic and the hedonic regulation of eating behavior (Di Marzo 2009). Cannabis has been known for a long time as increaser of appetite and food consumption (Kirkham 2005). This property can be explained by the characteristics of CB1 receptor. It acts in several mechanisms, such as central appetite control, peripheral metabolism, and body weight regulation (Kogan and Mechoulam 2007). In human organism and several other species CB1 agonists (e.g. THC) increase food intake and promote body weight gain, whereas selective CB1 receptor antagonists (e.g. cannabidiol) may reduce food intake and body weight (Izzo and Camilleri 2008). This phenomenon provides many possibilities to use natural or synthetic cannabinoids in many eating disorders, such as anorexia, bulimia, obesity or low body weight.
Oral cannabinoids, such as THC, CBD and nabilone, alone or in combination, have shown efficacy in treatment in central and peripheral neuropathic pain as well as rheumatoid arthritis and fibromyalgia (Ware et al. 2010). Clinical trials demonstrated significant analgesic effects by the cannabinoids studied. Another reported benefit was better sleep and in trials with multiple sclerosis patients also benefits in muscle stiffness and spasticity were found. Adverse effects related with cannabinoids were predominantly fatigue, dizziness, dry mouth, nausea and disturbances in cognition. These effects were mild to moderate, transient and generally well tolerated. Cited authors suggest that cannabinoids demonstrate a modest analgesic effect and are safe in the management of chronic pain (Lynch and Ware 2015).
Cannabinoids cause several cardiovascular effects. These compounds may involve modulation of the autonomic outflow in both the central and peripheral nervous systems as well as direct effects on the myocardium and vasculature. Acute effect of smoking cannabis in humans usually is linked with an increase in heart rate with no significant change in blood pressure. However, chronic marijuana uptake cause a long-lasting decrease in blood pressure and heart rate. The ability of decreasing blood pressure can be exploited in case of hypertensive patients. Vascular tissue also has CB1 receptors as well as the myocardium. Agonists, such as phyto- and endocannabinoids exert major hypotensive and cardiodepressor effects in vivo by the stimulation of these receptors. Scientific evidence suggests, that there is/are another yet-undefined endothelial and cardiac receptor/receptors that have ability to mediate some endocannabinoid-induced cardiovascular effects (Pacher et al. 2005).
Conclusions
The main obstacles limiting the use of marijuana in medicine are the psychotropic side effects and possible contraindications, such as euphoria, tolerance development, withdrawal symptoms, induction of psychosis or depression and impairments in cognitive function. These symptoms are the reason of fear of long-term therapy using cannabinoids. On the other hand, we have a lot of advantages and beneficial actions in human organism. Phytocannabinoids may be helpful in many disorders, such as epilepsy, Tourette syndrome, multiple sclerosis, eating disorders or chronic pain. This material is controversial in many spheres, such as pharmacologic, clinical, and societal. In order to find a solution of social dispute between the supporters and the opponents of medical marijuana legalization the researchers must cooperate. Full legalization of marijuana for medicine can be a difficult task to achieve. For this reason, future studies must concentrate at the development of orally administered, highly bioavailable, non-psychoactive phytocannabinoid products.
References & External links
- Anwar, Farooq, Sajid Latif, and Muhammad Ashraf. “Analytical characterization of hemp (Cannabis sativa) seed oil from different agro-ecological zones of Pakistan.” Journal of the American Oil Chemists’ Society 83.4 (2006): 323-329.
- Appendino, Giovanni, G. Chianese, and O. Taglialatela-Scafati. “Cannabinoids: occurrence and medicinal chemistry.” Current medicinal chemistry 18.7 (2011): 1085-1099.
- Ashton, John C., et al. “Expression of the cannabinoid CB2 receptor in the rat cerebellum: an immunohistochemical study.” Neuroscience letters 396.2 (2006): 113-116.
- Burstein, Sumner. “Cannabidiol (CBD) and its analogs: a review of their effects on inflammation.” Bioorganic & medicinal chemistry 23.7 (2015): 1377-1385.
- Callaway, J. C. “Hempseed as a nutritional resource: an overview.” Euphytica 140.1-2 (2004): 65-72.
- Di Marzo, Vincenzo, Maurizio Bifulco, and Luciano De Petrocellis. “The endocannabinoid system and its therapeutic exploitation.” Nature reviews Drug discovery 3.9 (2004): 771.
- Di Marzo, Vincenzo. “The endocannabinoid system: its general strategy of action, tools for its pharmacological manipulation and potential therapeutic exploitation.” Pharmacological research 60.2 (2009): 77-84.
- Galasso, Incoronata, et al. “Variability in seed traits in a collection of Cannabis sativa L. genotypes.” Frontiers in plant science 7 (2016): 688.
- Hemming, Mark, and Peter M. Yellowlees. “Effective treatment of Tourette’s syndrome with marijuana.” Journal of Psychopharmacology 7.4 (1993): 389-391.
- Howlett, A. C., et al. “International Union of Pharmacology. XXVII. Classification of cannabinoid receptors.” Pharmacological reviews 54.2 (2002): 161-202.
- Izzo, Angelo A., and Michael Camilleri. “Emerging role of cannabinoids in gastrointestinal and liver diseases: basic and clinical aspects.” Gut 57.8 (2008): 1140-1155.
- Kirkham, T. C. “Endocannabinoids in the regulation of appetite and body weight.” Behavioural pharmacology 16.5-6 (2005): 297-313.
- Kogan, Natalya M., and Raphael Mechoulam. “Cannabinoids in health and disease.” Dialogues in clinical neuroscience 9.4 (2007): 413.
- 14. Lazenka MF, Tomarchio AJ, Lichtman AH, Greengard P, Flajolet M, Selley DE et al. Role of dopamine type 1 receptors and dopamine-and cAMP-regulated phosphoprotein Mr 32 kDa in 9-tetrahydrocannabinol–mediated induction of FosB in the mouse forebrain. Journal of Pharmacology and Experimental Therapeutics. 2015; 354(3): 316-327.
- Leussink, Verena Isabell, et al. “Symptomatic therapy in multiple sclerosis: the role of cannabinoids in treating spasticity.” Therapeutic advances in neurological disorders 5.5 (2012): 255-266.
- Liang, Jingbang, Ayyappan Appukuttan Aachary, and Usha Thiyam‐Holländer. “Hemp seed oil: Minor components and oil quality.” Lipid Technology 27.10 (2015): 231-233.
- Lynch, M. E., and Mark A. Ware. “Cannabinoids for the treatment of chronic non-cancer pain: an updated systematic review of randomized controlled trials.” Journal of neuroimmune pharmacology 10.2 (2015): 293-301.
- Mechoulam, Raphael, and Linda A. Parker. “The endocannabinoid system and the brain.” Annual review of psychology 64 (2013): 21-47.
- Müller‐Vahl, K. R., et al. “Cannabinoids: possible role in patho‐physiology and therapy of Gilles de la Tourette syndrome.” Acta Psychiatrica Scandinavica 98.6 (1998): 502-506.
- Müller-Vahl, Kirsten R., et al. “Treatment of Tourette’s syndrome with Δ9-tetrahydrocannabinol (THC): a randomized crossover trial.” Pharmacopsychiatry 35.02 (2002): 57-61.
- Müller-Vahl, Kirsten R., et al. “Δ9-tetrahydrocannabinol (THC) is effective in the treatment of tics in Tourette syndrome: A 6-week randomized trial.” The Journal of clinical psychiatry (2003).
- Müller-Vahl, Kirsten R. “Treatment of Tourette syndrome with cannabinoids.” Behavioural Neurology 27.1 (2013): 119-124.
- Pacher, P., S. Batkai, and G. Kunos. “Cardiovascular pharmacology of cannabinoids.” Cannabinoids. Springer, Berlin, Heidelberg, 2005. 599-625.
- Pacher, P., and R. Mechoulam. “Is lipid signaling through cannabinoid 2 receptors part of a protective system?.” Progress in lipid research 50.2 (2011): 193-211.
- Pazos, M. Ruth, et al. “Mechanisms of cannabidiol neuroprotection in hypoxic–ischemic newborn pigs: Role of 5HT1A and CB2 receptors.” Neuropharmacology 71 (2013): 282-291.
- Pertwee RG. Pharmacological actions of cannabinoids. In: Pertwee RG, (red.). Cannabinoids. Berlin Heidelberg: Springer; 2005: 1-51.
- Piomelli, Daniele, et al. “The endocannabinoid system as a target for therapeutic drugs.” Trends in Pharmacological Sciences 21.6 (2000): 218-224.
- Patton, George C., et al. “Cannabis use and mental health in young people: cohort study.” Bmj 325.7374 (2002): 1195-1198.
- Pryce, G., and D. Baker. “Control of spasticity in a multiple sclerosis model is mediated by CB1, not CB2, cannabinoid receptors.” British journal of pharmacology 150.4 (2007): 519-525.
- Siudem P, Wawer I, Paradowska K. Konopie i kannabinoidy. Farmacja Współczesna. 2015; 8: 1-8 (in Polish).
- Tramèr, Martin R., et al. “Cannabinoids for control of chemotherapy induced nausea and vomiting: quantitative systematic review.” Bmj 323.7303 (2001): 16.
- Vonapartis, Eliana, et al. “Seed composition of ten industrial hemp cultivars approved for production in Canada.” Journal of Food Composition and Analysis 39 (2015): 8-12.
- Ware, Mark A., et al. “Smoked cannabis for chronic neuropathic pain: a randomized controlled trial.” Canadian Medical Association Journal 182.14 (2010): E694-E701.
- Welty, Timothy E., Adrienne Luebke, and Barry E. Gidal. “Cannabidiol: promise and pitfalls.” Epilepsy currents 14.5 (2014): 250-252.
The journal has had 7 points in Ministry of Science and Higher Education parametric evaluation. Part B item 1223 (26.01.2017).
1223 Journal of Education, Health and Sport eISSN 2391-8306 7
© The Authors 2017;
This article is published with open access at Licensee Open Journal Systems of Kazimierz Wielki University in Bydgoszcz, Poland
Open Access. This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited. This is an open access article licensed under the terms of the Creative Commons Attribution Non Commercial License (http://creativecommons.org/licenses/by-nc/4.0/) which permits unrestricted, non commercial use, distribution and reproduction in any medium, provided the work is properly cited.
The authors declare that there is no conflict of interests regarding the publication of this paper.
Received: 05.08.2017. Revised: 10.08.2017. Accepted: 31.08.2017.
The original text taken from a:
![]() http://www.ojs.ukw.edu.pl/index.php/johs/article/view/4876
http://www.ojs.ukw.edu.pl/index.php/johs/article/view/4876
Dąbrowski, Grzegorz, and Marta Skrajda. "Cannabinoids from Cannabis sp.: mechanism of their activity and potential health benefits in human body." Journal of Education, Health and Sport 7.8 (2017): 936-945.
Available under conditions of the license:![]() http://creativecommons.org/licenses/by-nc/4.0/
http://creativecommons.org/licenses/by-nc/4.0/











Comments
“Cannabinoids from Cannabis sp.: mechanism of their activity and potential health benefits in human body”