Department of Microbiology, Federal University of Technology, Akure, Ondo State, Nigeria. Department of Science Laboratory Technology, Osun State Polytechnic, Iree, Osun State, Nigeria. Department of Biological Sciences, Afe Babalola University, Ado-Ekiti, Ekiti State, Nigeria.
Fermentation is known to improve the nutritional value of food samples. This study was conducted to assess the influence of fermentation on bush mango in order to reduce environmental pollution. Bush mango seed cotyledons were naturally fermented for five days at room temperature.
The bacteria and fungi responsible for the fermentation were isolated and characterised using standard microbiological techniques. The unfermented seeds and fermented residues were analysed for proximate composition, nutrient composition and some antinutrient contents. The bacteria isolated from fermented bush mango seeds were Bacillus cereus, B. subtilis, Aerobacter aerogenes, Staphylococcus epidermidis, Streptococcus faecalis and Clostridium sporogenes. The fungal isolates included Penicillium chrysogenum, Aspergillus flavus, A. niger, Trichoderma viride and Rhizopus stolonifer. The results of the proximate analysis show that there was a decrease in fat content of the fermented seeds (65.30%) compared to unfermented seeds (56.13%) while the crude protein and nitrogen free extract increased from 0.54 to 2.25% and 19.71 to 31.08% respectively. The mineral contents (Mn, K, Fe) were found to be lower while P, Mn and Na were high. There was reduction in the phytate (from 1.40 to 0.67) and oxalate (1.26 to 0.72) contents after fermentation. Fermented bush mango seed cotyledons can be used as highly proteinous and low fat food condiment for soup making as well as animal feeds.
INTRODUCTION
Irvingia gabonensis is a non-timber forest product, made up of tree trunk (stem), leaves, roots and fruits. The fruit comprises a fleshy part and the nut, which consists of a hard shell and the kernel/seed. Its seeds have an outer brown testa (hull) and two white cotyledons (Ekpe et al., 2007). The fruit comprises a fleshy part and the nut, which consists of a hard shell and the kernel/seed. Its seeds have an outer brown testa (hull) and two white cotyledons. Two varieties have been identified in Nigeria; Var gabonensis and Var excelsa (Okafor and Ujor, 1994). It is an edible African indigenous fruit tree that produces edible fruits and seeds (Atangana et al., 2002). Common names of I. gabonensis include bush mango, African mango, wild mango or Dikanut plant. Irvingia seeds constitute an important part of the rural diet in Nigeria. The sun-dried seeds are ground into flour and used as soup thickeners (Ekpe et al., 2007). The white cotyledons are roasted and eaten in the Bwemba community of Uganda; roasted seeds confer flavour and aroma on foods especially vegetables (Ousseynou and Nicodeme, 1994). It is the food gum component of the seeds that serves as a thickening agent in water (Ndjouenkeu et al., 1996).
It has been noted that no single group or category of foods or food products are as important as fermented foods and have been relative to man’s nutritional well being throughout the world. Chemical compounds which are end products of fermentation process are tasty to many and are enjoyed by a large number of people of different ethnic groups (Ekundayo and Ojokoh, 2004). Fermentation is one of the oldest biotechnologies used in the enhancement of the nutrient content and preservation of food through the biosynthesis of vitamins, essential amino acids and proteins, by improving protein and fibre digestibility and by degrading antinutritional factors (FAO, 1983). Several food industries utilise microorganisms and the fermentation process in the preparation of foods (Ekundayo and Ojokoh, 2004). Fermentation process tends to reduce the toxicity of some foods for example gari and penjerim which are both from cassava (Namibisan and Sundaresan, 1985). The fermenting organisms include lactic acid bacteria, acetic acid producing bacteria and some alcohol producing yeasts. Certain mould species also play a very important role in some fermented foods (Frazier and Westholf, 1989). Much work has been done on fermentation of the seeds of I. gabonensis, however there is limited report on the fermentation of the cotyledons. The study was therefore aimed at investigating nutrient and chemical composition of liquid substrate fermented bush mango seed cotyledons.
MATERIALS AND METHODS
Fermentation of bush mango (I. gabonensis) seed cotyledons
The seeds of I. gabonensis were purchased at Bodija market Ibadan and put in polyethylene bag, which was directly brought to the laboratory for examination. The bush mango (I. gabonensis) seed cotyledons were surface sterilized by dipping in 90% ethyl alcohol for 1 min and rinsed in several changes of sterile distilled water. The seed cotyledons were then allowed to ferment naturally for 5 days.
Isolation of microorganisms
The filtrates of the fermented seeds were cultured on nutrient agar (Lab M) and potato dextrose agar (Lab M) after serial dilution for bacteria and fungi respectively. Bacterial isolates were identified and characterized based on their colonial morphology, cellular morphology and biochemical characteristics according to the method of Fawole and Oso (1988) while fungal isolates were identified using cultural and morphological features (Barnett and Hunter, 1972).
Proximate and mineral analyses of unfermented and fermented bush mango seed cotyledons
Moisture content was determined by drying to constant weight at 105°C in an oven, ash by ignition at 55°C in a muffle furnace, oil content by soxhlet extraction with hexane, protein by micro Kjedahl method and crude fibre by Acid/Alkali digestion methods (AOAC, 1990). The mineral were analyzed by dry ashing the sample at 55°C to constants weight and dissolving the ash in volumetric flask using distilled, de-ionized water with 10 ml of 10% hydrochloric acid solution. Sodium and Potassium were determined by using a flame photometer (model 405, corning UK) using NaCl and KCl salts to prepare the standards. All other metals were determined by Atomic absorption spectrophotometer (Perkin-Elmer model 403, Norwalk CT, USA). All chemicals used were of analytical grade. Earlier, the detection limit of the metal had been determined according to Techtron (1975). The optimum analytical grade was 0.1 to 0.5 absorbance unit with a coefficient of variance of variance of (0.87-2.20%) the mineral reported as mg/100 g. Phytate and tannin was determined using AOAC (1990) methods while oxalate content was by the titrimetric method as modified by Akindahunsi and Salawu (2005).
RESULTS AND DISCUSSION
A total of 11 microorganisms were isolated from fermented seed filterate of bush mango seed cotyledons. Six of the above microorganisms were bacteria while the remaining five were fungi. The bacteria isolated were Bacillus cereus, Aerobacter aerogenes, Staphylococcus epidermidis, Streptococcus faecalis, Clostridium sporogenes and Bacillus subtilis. The fungi isolated were Penicillium chrysogenum, Aspergillus flavus, A. niger, Trichoderma viride and Rhizopus stolonifer.
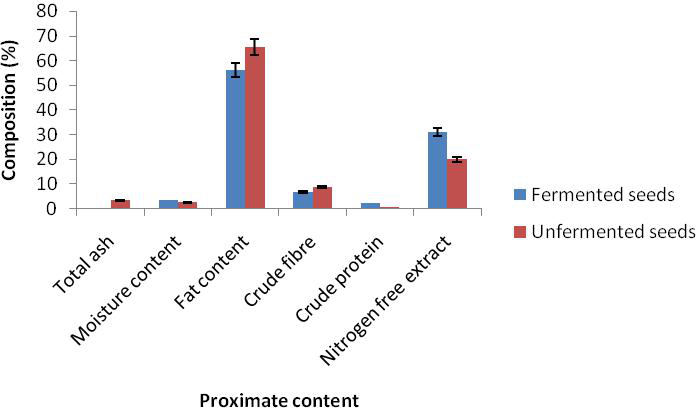
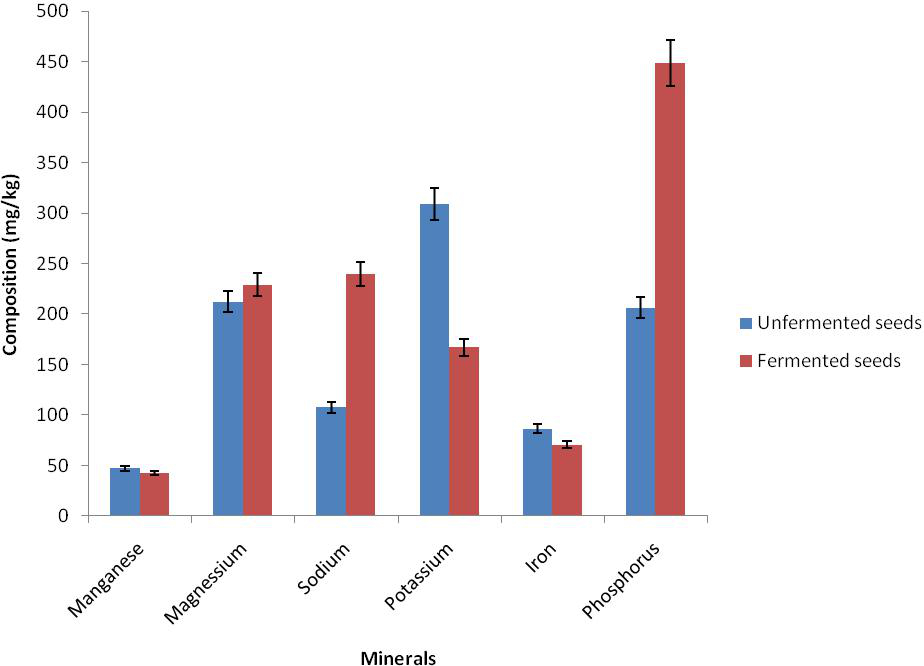
The result of proximate composition of bush mango seed showed that there was reduction in ash content from 3.30 to 0.43%, fat from 63.00 to 56.13%, and crude fibre from 8.73 to 6.69% while there was an increase in dry matter (moisture content) from 2.42 to 3.42%, crude protein from 0.54 to 2.25% and nitrogen free extractives from 19.71 to 31.08% in the unfermented and the fermented mango seed (Figure 1). There was also a reduction in antinutrient in phytate from 1.40 to 0.67 and oxalate 1.26 to 0.72 while tannin increased from 0.395 to 1.08 respectively (Figure 2). There was an increase in magnesium from 212.29 to 229.20, calcium from 107.63 to 239.56, sodium from 101.68 to 166.68 and phosphorus from 206.18 to 448.70 while reduction occured in potassium from 86.30 to 70.31 in the unfermented and fermented seed respectively (Figure 3).
Figure 1. Proximate composition of fermented and unfermented seed cotyledons of bush mango
Figure 2. Antinutrient composition of fermented and unfermented seed cotyledons of bush mango
Figure 3. Mineral composition of fermented and unfermented seed cotyledons of bush mango
An array of microorganisms was isolated during the fermentation of bush mango seed cotyledons which is a testament to the ubiquity of microorganisms. The work of Bergman et al. (1981) revealed the presence of Streptococcus lactic in maize during the production of “Ogi” and “Agidi” which is also a fermented food and it was also isolated in this research work from fermented bush mango seed cotyledons. S. epidermidis and B. subtilis that were isolated in bush mango seed cotyledons were found in the fermentation of Parkia biglobosa (Locust beans seed) in the production of “Iru” a food condiment (Diawara, 2000). Fungi isolated from the seed cotyledons are similar to those isolated by Aboloma and Ogunbusola (2012). The work of Ojokoh (2007) showed the presence of Aspergillus and Rhizopus in the fermenting mango peel as also isolated from the bush mango seed cotyledons.
Traditional societies have always exploited edible wild plants to provide adequate nutrition, food security and income generation (Akubugwo and Ugbogu 2007; Akubugwo et al., 2008). The proximate composition of bush mango seed showed that there was reduction after fermentation in ash, fat and crude fibre content while there was increase in dry matter, crude protein and nitrogen free extractives. The increase in the moisture content could be attributed to the addition of water to the sample prior to fermentation as also observed by Ojokoh et al. (2012). Reduction in fat content is advantageous to prevent excess fat in the body. Ekpe et al. (2007) also observed a decrease in the crude fat of I. gabonensis as a result of processing. Since the ash content is a measure of the total amount of minerals present within a food, a reduction in its level during microbial fermentation could be as a result of the minerals being used up by the fermenting organisms as a mineral source during their metabolism (Aderiye and Ogunjobi, 1998).The increase in protein content of the seed is in line with the work of Fadare and Ajaiyeoba (2008). The high protein contents could be attributed to the ability of microorganisms to secret some extra cellular enzymes capable of degrading cellulolytic materials during fermentation (Oseni and Ekperigin, 2007). The increase in protein level could also be attributed to increased microbial nitrogen during fermentation as a result of increased production of single cell proteins (Ekpe et al., 2007).
The antinutrient composition of bush mango seed showed that phytate and oxalate content reduced after fermentation while tannin content increased which is an agreement with the work of Oseni and Ekperigin (2007). A wide range of microflora has been known to possess phytase activity (Ojokoh, 2005) which may be partly responsible for reduction in phytic acid content in the fermenting samples. Perez-Hidalgo et al. (1997) proposed that the formation of resistant starch together with condensed tannin-protein content may be responsible for the increase in tannin content. However, reduction in tannin of fermented products such as Jack beans and African oil bean seed were observed by Odetokun, (2000) and Enujiugha and Akanbi (2005).
The result of nutrient composition showed that magnesium, calcium, sodium and phosphorus increased after fermentation of bush mango seed. However, manganese, potassium and iron content were reduced. The fermented sample was rich in some essential minerals which perform various functions in the body (Akindahunsi et al., 1999).
In conclusion, bush mango seed cotyledons have nutritional benefits which can be enhanced when fermented. Fermentation helps to increase the protein and nitrogen free extractives of the seeds thus improving its nutritional quality. There was also reduction in the fat content, which is of good advantage to the body. It is then recommended that bush mango seed cotyledons be fermented before consumption due to increase nutritional composition after fermentation.
References & External links
- Aboloma RI, Ogunbusola EM (2012). Fungi associated with Irvingia gabonensis (Ogbono) and Colocynthis citrullus(Egusi) seeds sold in markets in Ado-Ekiti , Ekiti State Nigeria. J. Nat. Prod. Plant Resour. 2(3):423-426.
- Adebayo – Tayo BC, Onilude AA, Ogunjobi AA, Gbolagade JS, Oladapo MO (2006). Detection of fungi and aflatoxin in shelved bush mango seeds (Irvingia spp.) stored for sale in Uyo, Nigeria. Afr. J. Biotech. 5 (19):1729-1932.
- Aderiye BJ, Ogunjobi AA (1998). Fermentation of Yam, microbiology and sensory evaluation of cooked fermented yam tissues. Intl. J. Plant Food Human Nutr. 52: 49-54.
- Akindahunsi AA, Oboh G, Oshodi AA (1999). Effect of fermenting cassava with Rhizopus oryzae on the chemical composition of its flour and gari, La Rivista Italiana Delle Sostanze Grasse 76: 437-440.
- Akindahunsi AA, Salawu SO (2005). Phytochemical screening and nutrient antinutrient composition of selected tropical green leafy vegetable. Afr. J. Biotech. 4:6.
- Akubugwo IE, Chinyere GC, Ugbogu AE (2008). Comparative studies on oils from some common plant seeds in Nigeria. Pak. J. Nutr. 7:570-573.
- Akubugwo IE, Ugbogu AE (2007). Physicochemical studies on oils from five selected Nigerian plant seeds. Pak. J. Nutr. 6:75-78.
- Association of official analytical chemist (1990). Official methods of analysis, 15 ed, Washington DC, USA.
- Atangana AR, Tchoundjeu Z, Foldout JM, Asaah E, Dumb M, Leakey RRB (2001). Domestication of Irvinga gabonesis: 1 phenotypic variation in fruit and kernels in two populations from Cameroon. Agrofor. Syst. 53:55-64.
- Bannet HL, Hunter BB (1972). Illustrated Genera of Imperfect Fungi. Minneapolis Burgress Publishing Company. Minneapolis, MN. p. 241.
- Bergmann E (1981). Nutzungsmospglick eaten moderner erkenntnisse der biotechnologic fur entwicklungslander for schungsberichte des BMZ, band 18. weltforum verlag. koln.
- Diawara A (2000). African locust bean preparation. J. Plants Foods 4:245-263.
- Ekpe OO, Umoh IB, Eka OU (2007). Effect of a typical rural processing method on the proximate composition and amino acid profile of bush mango seeds Iirvingia gabonensis). African J. Food. Agric. Nut. Dev. 7 (1):1-12.
- Ekundayo FO, Ojokoh AO (2004). Improvement in the proximate and nutrient values of sweet potato after microbial fermentation. J. Sci. Tech. Res. 3 (4):1-3.
- Enujiugha VN, Akanb CT (2005). Compositional changes in African oil bean (Pentaclethra macrophylla Benth) seeds during thermal processing. Pak. J. Nutr. 4(1):27-31.
- Fadare DA, Ajaiyeoba EO (2008). Phytochemical and antimicrobial activities of the wild mango – Irvingia gabonensis extracts and fractions. Afri. J. Med Sci. 37(2): 119 -124.
- FAO (1983). Food and fruit breeding forest species. In: Examples from East Africa. FAO Forestry. 44(1):172.
- Fawole MO, Oso BA (1988). Laboratory Manual of Microbiology. Published by spectrum books limited, Ibadan, Nigeria. 5:22-33.
- Frazier WC, Westhoff DC (1989). Foodborne infection and intoxication. Bacterial in food microbiology. 2nd edition, Tata McGraw Hill Publishing Company Limited, New Delhi. p. 450.
- Namibisan B, Sundaresan S (1985). Effect of and processing on the cyanoglucoside in cassava. J. Sci. Food Agric. 36:1197.
- Ndjouenkeu R, Goycoolea FM, Morris ER, Akingbala JO (1996). Okra (hibiscus esculentus) dika nut (Irvingia gabonensis) polysaccharides. Car. Polym. 29:263-269.
- Odetokun SM (2000). Effect of fermentation on some physiochemical properties, antinutrients and in vitro multienzyme digestibility of selected legumes. Ph.D thesis. Federal University of Technology, Akure, Nigeria.
- Ojokoh AO (2005). Effect of fermentation on the nutritional qualities of roselle (Hibiscus sabdariffa Linn) calyx Ph.D thesis, Federal University of Technology, Akure, Nigeria.
- Ojokoh AO (2007). Effect of fermentation on the chemical composition of mango (Mangifera indica) peels. Afri. J. Biotech. 6: 1979-1981.
- Ojokoh AO, Abiola AB, Lawal RT (2012). Changes in nutrient and antinutrient composition of popcorn and groundnut composite flour subjected to solid substrate fermentation. Afr. J. Agric. Res. 7(23):3439-3445.
- Okafor JC, Ujor G (1994). Varietal differences in Irvingia gabonensis. Paper Presented at the ICRAF Pre-collection meeting, IITA Ibadan 5.
- Oseni OA, Ekperigin M (2006). Studies on biochemical changes in maize wastes: fermented with Aspergillus niger. Biokemistri 19(2):75-79.
- Ousseynou N, Nicodeme T (1994). International Institute of Tropical Agriculture and the Rockefeller Foundation. A paper on Utilization and marketing of Irvingia gabonensis produce in the humid forest of Cameroon 80.
- Perez-Hidalgo MA, Guerra-Hernendez E, Garcia-Villanova B (1997). Dietary fiber in three raw legumes and processing. Effect on chick peas by an enzymatic gravimetric method. J. Food Composition and Analysis 10: 66-72.
- Techtron V (1975). Basic atomic spectroscopy: A modern introduction: Dominican press Victoria, Australia. pp. 104-106.
The original text taken from a:
![]() http://www.academicjournals.org/journal/AJMR/article-abstract/904D54C13612
http://www.academicjournals.org/journal/AJMR/article-abstract/904D54C13612
Ekundayo, F. O., Oladipupo, O. A. and Ekundayo, E. A.. "Studies on the effects of microbial fermentation on bush mango (Irvingia gabonensis) seed cotyledons." African Journal of Microbiology Research 7, no. 34 (2013): 4363-4367.
Available under conditions of the license:![]() http://creativecommons.org/licenses/by/4.0/
http://creativecommons.org/licenses/by/4.0/











Comments
“Studies on the effects of microbial fermentation on bush mango (Irvingia gabonensis) seed cotyledons”